Excellent Resistance to Corrosion and Bio Fouling in Sea WaterCopper-nickel alloys have high resistance to sea water environments. There are two common alloys, containing nominally 10% and 30% of nickel. The 10% nickel alloy is the most widely used for sea water handling service. Chemical CompositionASTM B466 Seamless copper nickel pipe & tube | Alloy | Name | Copper | Nickel | Iron | Lead | | C70600 | 90-10 copper nickel | ~89 | 9.0-11.0 | 1.0-1.8 | <0.05 | | C71500 | 70-30 copper nickel | ~69 | 29.0-33.0 | 0.4-1.0 | <0.05 |
Sea Water Pipe Work | Copper-nickel is an established pipe work alloy for many of the world’s navies.It is also used in merchant shipping, the power industry and offshore for a variety of purposes. |
Offshore Fire Water Systems | Sea water deluge fire extinguishing systems in copper-nickel have been selected for offshore platforms over many years. | |
Heat Exchangers and Condensers | Good thermal conductivity and corrosion resistance to the sea water flow rates required have allowed copper-nickel tubing to remain an established alloy where high reliability is called for. |
Sea Water Intakes | Fouling on intakes and intake screens can restrict water flow and if detached cause blockages to heat exchangers or cause mechanical damage to pumps and valves. Copper-nickel with its high resistance to macrofouling can be very beneficial in this application. | Photo: International Copper Research Association | |
Fish Farming | The excellent bio-fouling and corrosion resistance of 90/10 copper-nickel mesh coupled with its mechanical strength and low resistance to water flow, make it an ideal material for the large scale development of underwater pens and enclosures. thus adding a new dimension to fish farming. | |
Sheathing of Legs and Risers on Offshore Platforms | Copper-nickel is used for splash zone corrosion protection on oil/gas platform legs and riser pipes either as welded sheet or as a composite with neoprene. In an insulated form it provides antifouling properties too, which reduces wave drag and cleaning operations on the structures. More novel applications include sub-sea markers made from copper-nickel wire gauze which remain fouling free to help divers identify submerged structural areas. |
Boat HullsCopper-nickel is one of the few engineering materials with good inherent resistance to both corrosion and biofouling making the alloy a suitable material for boat hulls without recourse to cathodic protection or antifouling and anticorrosion paints. |
Hydraulic LinesWith adequate strength to withstand pressures in most marine hydraulic and instrumentation systems, copper-nickel provides good service, combined with ease of manipulation at installation. |
Desalination UnitsConsiderable quantities of 90-10 copper-nickel are used in Multi-Stage Flash Desalination Units predominately located in the Middle East. The alloy is a prime condenser tubing material for the Heat Recovery Section and is also used for water boxes, tube plates and other fabrications as solid material or as clad steel plate. |
Alloy Properties | | Low General Corrosion Rates in Sea WaterGeneral corrosion rates are normally in the order of 0.0025-0.025mm/yr, which makes the alloy suitable for requirements in most marine applications. | | Resistance to Stress Corrosion Cracking Due to Ammonia in Sea Water Copper based alloys (e.g. brass) can be susceptible to ammoniacal stress corrosion cracking. However copper-nickel has the highest resistance to this and stress corrosion in sea water is not known to be a problem. | | High Resistance to Crevice Corrosion & Stress Corrosion due to ChloridesCopper-nickel is not susceptible to the type of crevice corrosion and stress corrosion cracking found in stainless steels. As such there is not a related temperature limitation for use in chloride environments. | | Good Pitting Resistance The resistance to pitting in clean sea water is good and if pits do occur they tend to be broad and shallow in nature rather than undercut. |
Readily Weldable and No Post Weld Heat Treatment Required | Copper-nickel is straightforward to weld by conventional welding techniques.The alloy can also be welded to steel. |
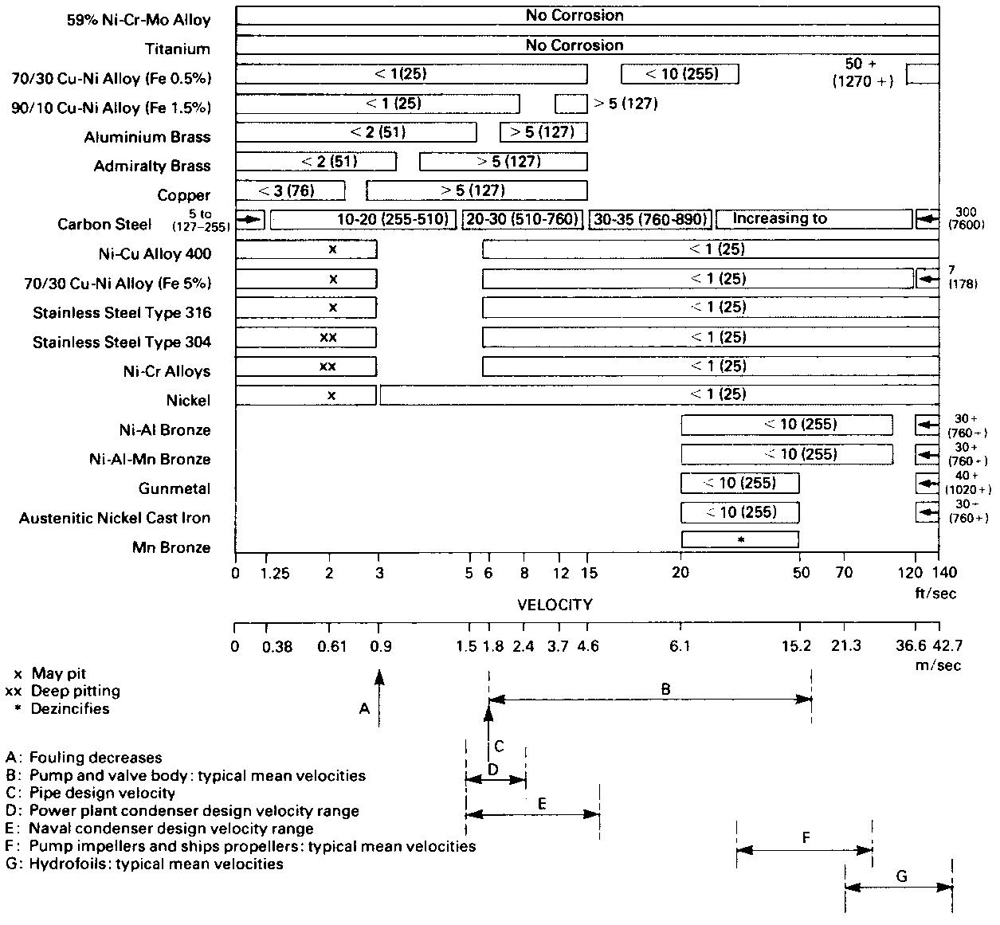
Mechanism of Corrosion ResistanceThe corrosion resistance of the alloys is due to the protective surface film formed when in contact with water. On initial immersion cuprous oxide is formed but complex changes occur in sea water which research work is only now beginning to elucidate. At a flow rate of 0.6 m/s the equilibrium corrosion rate is an almost negligible 0.002 mm/year. Normally, design flow rates of up to 3.5 m/s give a satisfactory safety factor for use in pipework systems. This figure makes allowance for the fact that local speeds may be higher at changes of direction, points of divergence, etc. If water velocity is excessive, it can cause vortices leading to impingement attack which can cause premature failure. Where surfaces in contact with water allow smooth flow, as in ships hulls, different design criteria apply. As mentioned, the fouling resistance is due to the copper ions at the surface, making it inhospitable to most marine organisms in slowly moving water. In static conditions there may be some deposition of chemical salts and biological slimes, possibly leading to some weakly adherent fouling but such residues are easily detached from the metal’s corrosion resistant surface, exposing a fresh, biocidally active surface. | Easy to FabricateHot and cold working techniques can be used but because of the good ductility of the alloy, cold working is normally preferred. | Sea Water Piping Systems can last a Ship’s LifetimeExperience over the last 40 years has confirmed the durability of copper-nickel. | Inherent Resistance to BiofoulingThe protective oxide surface film which forms naturally on CuNi in sea water also provides an inhospitable surface to deter marine growth. | Corrosion in Sea WaterThe 90/10 and 70/30 cupronickel alloys both have excellent resistance to bio-fouling and corrosion in sea-water with some variations in the performance of the alloys under different conditions as shown in Tables 10 and 11. For example, the 90/10 alloy has better bio-fouling resistance. The corrosion resistance of the 90/10 and 70/30 alloys in heat exchangers and condensers is compared with a number of other alloys in Figure 1. Table 7 gives the relative resistance of various alloys to fouling in quiet sea-water. If water velocity is accelerated above 1 m/sec, any slight bio-fouling on metal with good fouling resistance will be easily detached and swept away. On a material that does not have this good fouling resistance, strongly adherent, marine organisms would continue to thrive and multiply. | Design Conditions for Sea Water ServiceThe effect of water velocity on fouling and corrosion rates of various metals is shown in Figure 1, which also shows the typical service design speeds for some items of common equipment in contact with sea-water. The excellent corrosion resistance of 70/30 and 90/10 copper nickel alloys and their suitability for many applications can be seen. Some materials with apparently better corrosion resistance may have disadvantages such as lack of resistance to bio-fouling, lack of availability in the forms required, or susceptibility to crevice corrosion. They may also be more expensive and therefore less cost-effective over the required service lifetime. | Crevice Corrosion and BiofoulingCrevice corrosion can occur in components in sea-water when they are locally starved of oxygen at a joint or under attached bio-fouling. Table 8 shows the good tolerance of the copper-nickel alloys to this type of attack, giving these alloys advantages over other materials of equal corrosion resistance. The copper-nickel alloys have good corrosion resistance in the quiescent or stagnant conditions which may occur during the commissioning or overhaul of plant. Where plant is not being used at design speeds some other materials may fail. | Development of a Corrosion Resistant SurfaceWhen first brought into use, care must be taken to allow copper-nickel alloys to form their protective corrosion resistant surface freely. Normally, this protective film will develop in six to eight weeks. Contact with other less noble metals or with cathodic protection systems must be avoided to ensure development of the corrosion resistant surface film and the non-fouling properties. | Stress Corrosion CrackingCopper-nickel alloys do not suffer the stress-corrosion problems associated with some other materials, such as copper zinc alloys (brass) with more than 15% zinc. |
Specified Minimum PropertiesASTM B111 Copper & copper alloy seamless condenser tubes & ferrule stock | Temper | Code | 0.5% Proof Stress | Tensile Strength | Elongation | | MPa | MPa | % | | Annealed | O61 | 105 | 275 | (40) | | Cold Drawn | H55 | 240 | 310 | (10) |
Physical Properties| Property | Metric Units | Imperial Units | Melting Point (Liquidus) | 11,500°C | 21,000°F | Melting Point (Solidus) | 11,000°C | 20,100°F | | | | Density | 8.94 gm/cm³ @ 20°C | 0.323 lb/in³ @ 68°F | Specific Gravity | 8.94 | 8.94 | | | | Coefficient of Thermal Expansion | 17.1 x 10 -6 / °C (20-300°C) | 9.5 x 10 -5 / °F (68-392°F) | Themal Conductivity | 40 W/m. °K @ 20°C | 23 BTU/ft³/ft/hr/°F @ 68°F | Thermal Capacity (Specific Heat) | 380 J/kg. °K @ 20°C | 0.09 BTU/lb/°F @ 68°F | | | | Electrical Conductivity (Annealed) | 5.26 microhm?¹.cm?¹ @ 20°C | 9.1% IACS | Electrical Resistivity (Annealed) | 0.190 microhm.cm @ 20°C | 130 ohms (circ mil/ft) @ 68°F | | | | Modulus of Elasticity (tension) | 140 GPa @ 20°C | 20 x 10 6 psi @ 68°F | Modulus of Rigidity (torsion) | 52 GPa @ 20°C | 7.5 x 10 6 psi @ 68°F |
Fabrication Properties| Joining Technique | Suitability | Soldering | Excellent | Brazing | Good | Oxy Acetylene Welding | Not Recommended | Gas Shielded Arc Welding | Excellent | Coated Metal Arc Welding | Good | Resistance Welding | Good |
| | | Fabrication Technique | Suitability | Capacity for Being Cold Worked | Excellent | Capacity for Being Hot Worked | Good | Hot Working Temperature | 850 – 950 °C | Annealing Temperature | 700 – 825 °C | Stress Relieving Temperature | 275 – 400 °C | Machinability Rating | 20% of free cutting brass | Polishing/Electroplating Finish | Excellent |
|
ASTM Product Specifications | Title | | B469 | Seamless Copper Alloy Tubes for Pressure Applications | | B466 | Seamless Copper Nickel Pipe and Tube | | B552 | Seamless & Welded Copper Nickel Tubes for Water Desalting | | B543 | Welded Copper & Copper Alloy Heat Exchanger Tube | | B608 | Welded Copper Alloy Pipe | | B467 | Welded Copper Nickel Pipe | | B151 | Copper Nickel Zinc Alloy (Nickel Silver) & Copper Nickel Rod & Bar | | B111 | Copper & Copper Alloy Seamless Condenser Tubes & Ferrule Stock | | B359 | Copper & Copper Alloy Seamless Condenser & Heat Exchanger Tubes with Integral Fins | | B171 | Copper Alloy Plate & Sheet for Pressure Vessels, Condensers & Heat Exchangers | | B122 | Copper Nickel Tin Alloy, Copper Nickel Zinc Alloy (Nickel Silver), & Copper Nickel Plate, Sheet, Strip & Rolled Bar |
Corrosion Rates of Materials in Flowing Sea WaterApproximate corrosion rates are given by the figures on the bars in units/hr (micrometres/year, 1,000 micrometres = 1 mm)
 Fouling Resistance of Various Alloys in Quiet Sea Water| Arbitrary Rating Scale of Fouling Resistance | Materials | | 90-100 | Best | Copper | | 90/10 Copper Nickel Alloy | | | | | 70-90 | Good | Brass & Bronze | | | | | 50 | Fair | 70/30 Copper Nickel Alloy | | Aluminium Bronzes | | Zinc | | | | | 10 | Very Slight | Monel 400 (Nickel Copper Alloy) | | | | | 0 | Least | Carbon and Low Alloy Steels | | Stainless Steels | | Nickel Chromium Molybdenum Alloys | | Titanium |
Tolerance for Crevice Corrosion and Pitting Under Fouling in SeawaterCrevices can normally be tolerated in designs using these materials. They may foul but rarely pit. | Titanium Hastelloy C Inconel 625 | Titanium will pit at temperatures above 120°C. Inconel 625 after 2-3 years shows signs of incipient pitting in some tests in quiet seawater | | 90/10 copper-nickel(1.5 Fe)Admiralty Brass | Shallow to no pitting90/10 copper-nickel is standard seawater piping alloy. | | 70/30 copper-nickelCopperTin and aluminium bronzesAustenitic nickel cast iron | Good resistance to pittingUseful in piping applications | | | | Useful although cathodic protection required on critical surfaces | Monel 400 (nickel-copper alloy) | Pits tend to be self-limiting in depth at about 1-6 mm.No protection required for heavy sections.Cathodic protection from steel or copper base alloys will prevent pitting on O Ring, valve seats, and similar critical surfaces. | | CN7M (Alloy 20)Incoloy 825 | Occasional deep pits will develop.Protection not normally required for all alloy 20 pumps.Cathodic protection from less noble alloys may be necessary for O Ring and similar critical surfaces. | | Grade 316 Stainless Steel | Cathodic protection from zinc, aluminium, or steel is required except when part is frequently removed from seawater and thoroughly cleaned | | | | Crevices cannot be tolerated in designs (But usable in above the waterline marine applications) | Nickel | Many deep pits develop.Cathodic protection from less noble alloys required | | Grade 304 Stainless Steel | Many deep pits develop.Cathodic protection from steel may not be fully effective | | Precipitation Hardening Grades of Stainless Steel | Many deep pits develop.Cathodic protection with zinc or aluminium may induce cracking from hydrogen | | | | Severe crevice corrosion limits usefulness | Grade 303 Stainless Steel | Severe pitting.Cathodic protection may not be effective. | | Series 400 (ferritic or martensitic) Stainless Steel | Severe pitting.Cathodic protection with zinc or aluminium may induce cracking from hydrogen. |
Demonstration of Fouling Resistance | Panels from 55 week exposure trials conducted at Langstone Harbour,Portsmouth, UK, by IMI Yorkshire Alloys LtdPanels are, left to right:- carbon steel
- copper-nickel cladding on steel
- copper-nickel panels with aluminium anodes
- freely exposed copper-nickel (far right)
|
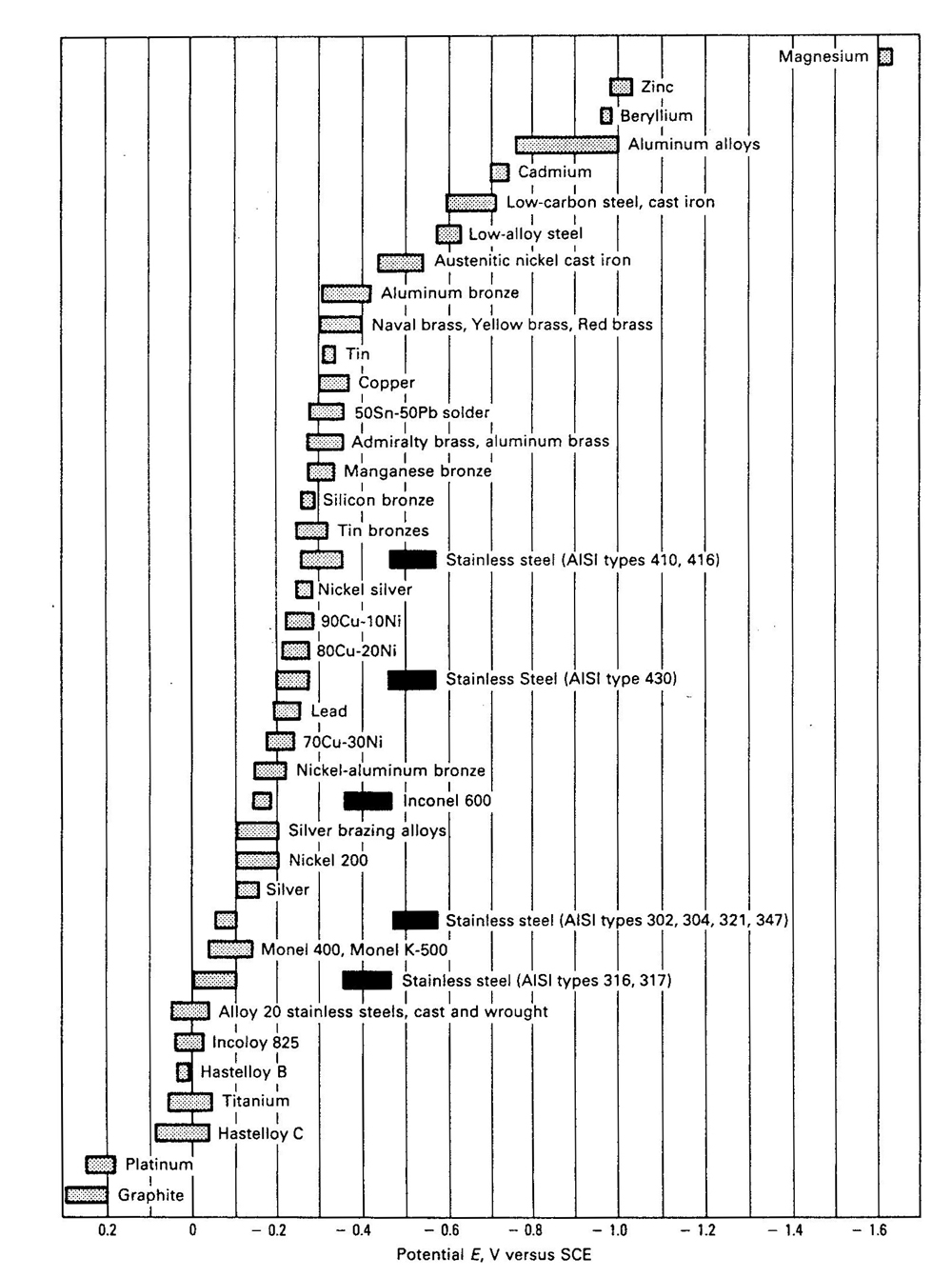
Corrosion Potentials in Sea WaterFlowing (2.5 – 4 m/sec) sea water at temperatures in the range 10 – 26°C. The solid bars indicate the potential of stainless steels actively corroding, e.g. in acidic water such as may exist in crevices. The shaded bars for stainless steels indicate behaviour in the presence of a passive film. 
Comparison of Corrosion Behaviourof CuNi10Fe and CuNi30Fe in Sea Water (in Heat Exchanger Service) Environmental Conditions | Type of Corrosion | Service Experience | CuNi10Fe | CuNi30Fe | Waterside Conditions | | | | Clean Seawater at velocities up to 1m/sec | Uniform, General | 0.0025-0.025 mm/yr | 0.0025-0.025 mm/yr | Clean Seawater at velocities up to 3.5 m/sec* | Impingement Attack | Satisfactory | Satisfactory | Polluted Seawater | Accelerated General & Pitting | Less Resistant | Preferred but not immune | Entrained Sand in Seawater | Accelerated General & Erosion | Unsuitable, except in mild conditions | Use CuNi30Fe2Mn2 | Accumulated Deposits on Surface | Local Attack | Generally Good | Tendancy to Pit | Hot Spots due to Local Overheating | Local Attack by Denickelification | Good | Good but some failures in extreme conditions | Corrosion Plus Stress | Stress Corrosion | Very Resistant | Very Resistant | Vapour Side Conditions | | | | Feedwater Heaters working under cyclic conditions | Exfoliation Attack | Resistant | Susceptible | Non-condensable gases † | Local Attack & General Thinning | Highly Resistant | Most Resistant | Hydrogen Sulphide in Desalination Plant | General Attack | Less Resistant | Resistant ‡ |
† lf concentration of CO2 is extremely high, stainless steel may be better cholce. ‡ Attack will increase in concentration or temperature. Design DataAllowable design stresses are given in:
AS1210 – 1997, Amendment No 2, September 1998 Pressure Vessels. Maximum metal temperature 300°C for 90/10 copper nickel, 375°C for 70/30 copper nickel.
AS4041 – 1998. Maximum metal temperature 325°C for 90/10 copper nickel, 425°C for 70/30 copper nickel. Corrosion of Copper Nickel Alloys| Medium | 90-10 Copper Nickel | 70-30 Copper Nickel | | Medium | 90-10 Copper Nickel | 70-30 Copper Nickel | Acetic Acid | B | B | | Freon | A | A | Acetic anhydride | B | B | | Fuel Oil | A | A | Acetone | A | A | | Hydrocarbons (pure) | A | A | Alcohols | A | A | | Hydrochloric acid | C | C | Aluminium chloride | B | B | | Hydrofluoric acid | C | B | Aluminium hydroxide | A | A | | Hydrogen peroxide | B | B | Aluminium sulphate | B | A | | Hydrogen sulphide (dry) | A | A | Ammonia (absolutely dry) | A | A | | Hydrogen sulphide (moist) | D | C | Ammonia (moist) | D | C | | Magnesium chloride | B | B | Ammonium hydroxide | D | C | | Magnesium hydroxide | A | A | Ammonium chloride | D | C | | Magnesium sulphate | A | A | Ammonium sulphate | D | C | | Methyl chloride (dry) | A | A | Aniline dyes | C | C | | Nitric acid | D | D | Barium chloride | B | B | | Paraffin | A | A | Barium hydroxide | A | A | | Phosphoric acid | B | B | Benzol | A | A | | Potassium carbonate | A | A | Bleaching powder (wet) | B | B | | Potassium chloride | A | A | Boric acid | A | A | | Potassium dichromate (acid) | D | D | Brines | A | A | | Potassium hydroxide | A | A | Bromine (dry) | A | A | | Seawater | A | A | Bromine (moist) | B | B | | Sewage | A | A | Butane | A | A | | Silver salts | D | D | Calcium bisulphate | B | B | | Sodium bicarbonate | A | A | Calcium chloride | A | A | | Sodium bisulphate | A | A | Calcium hydroxide | A | A | | Sodium bisulphate | A | A | Carbon dioxide (dry) | A | A | | Sodium carbonate | A | A | Carbon dioxide (moist) | B | B | | Sodium chloride | A | A | Carbon disulphide | B | B | | Sodium cyanide | D | D | Carbon tetrachloride (dry) | A | A | | Sodium dichromate (acid) | D | D | Carbon tetrachloride (moist) | B | A | | Sodium hydroxide | A | A | Chlorine (dry) | A | A | | Sodium hypochlorite | C | B | Chlorine (moist) | C | B | | Sodium nitrate | A | A | Chromic acid | D | D | | Sodium peroxide | B | B | Citric acid | A | A | | Sodium sulphate | A | A | Copper chloride | C | C | | Sodium sulphide | C | B | Copper sulphate | B | B | | Steam | A | A | Crude oil | B | A | | Sulphur dioxide (dry) | A | A | Ethers | A | A | | Sulphur dioxide (moist) | C | C | Ethyl acetate | A | A | | Sulphuric acid | B | B | Ethyl chloride | B | B | | Sulphurous acid | C | C | Ethylene glycol | A | A | | Tannic acid | A | A | Ferric chloride | D | D | | Trichloroethylene (dry) | A | A | Ferric sulphate | D | D | | Trichloroethylene (moist) | B | A | Ferrous chloride | B | B | | Zinc chloride | C | C | Ferrous sulphate | B | B | | Zinc sulphate | B | B |
B = the alloy has god corrosion resistance C = the alloy has fair corrosion resistance D = the alloy is not suitable |